Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial - The Lancet

Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial - The Lancet
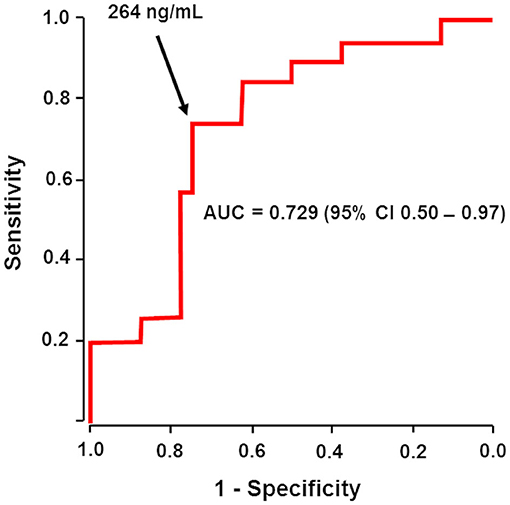
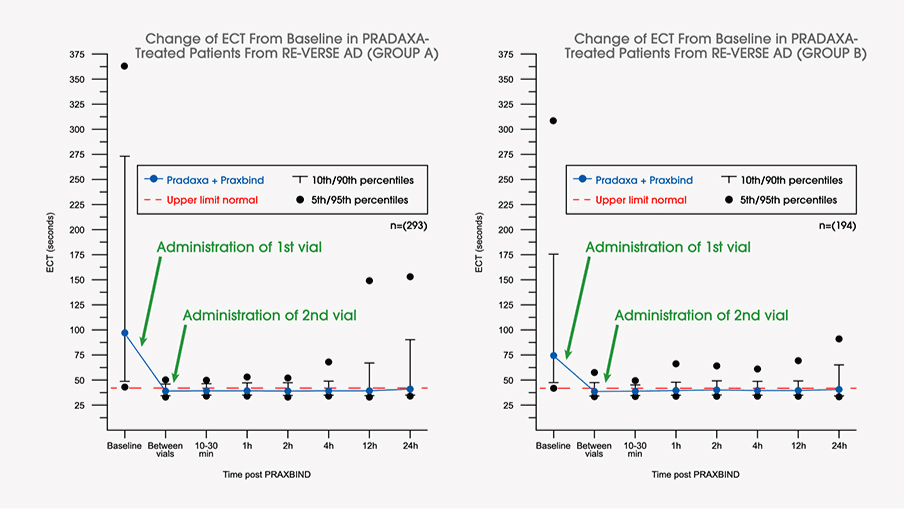
Frontiers | Dabigatran Level Before Reversal Can Predict Hemostatic Effectiveness of Idarucizumab in a Real-World Setting
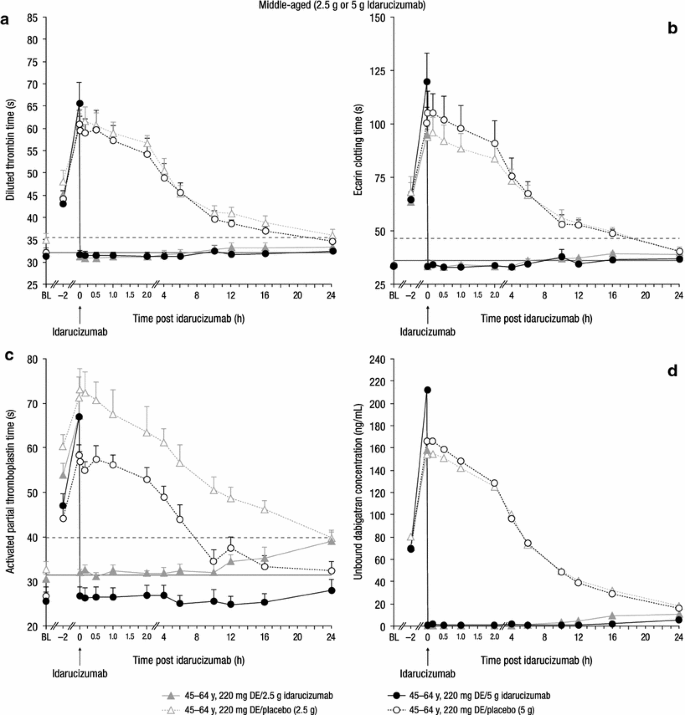
Effect of Age and Renal Function on Idarucizumab Pharmacokinetics and Idarucizumab-Mediated Reversal of Dabigatran Anticoagulant Activity in a Randomized, Double-Blind, Crossover Phase Ib Study | SpringerLink

Idarucizumab dosing in patients with excessive dabigatran body burden - British Journal of Anaesthesia

Idarucizumab for dabigatran reversal: the first 6 months in a tertiary centre - Wheeler - 2019 - Internal Medicine Journal - Wiley Online Library
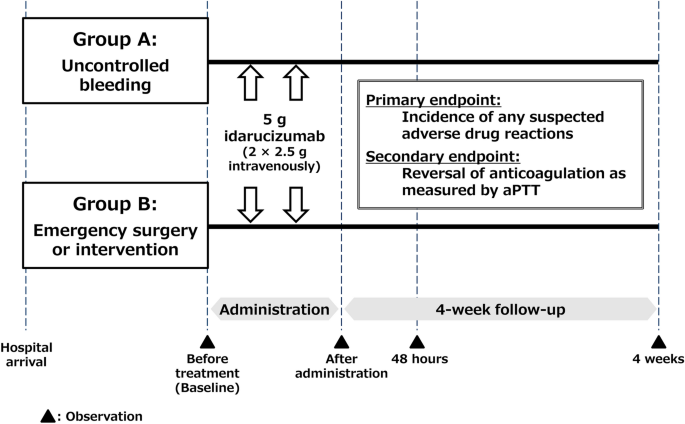
Idarucizumab for Emergency Reversal of Anticoagulant Effects of Dabigatran: Interim Results of a Japanese Post-Marketing Surveillance Study | SpringerLink
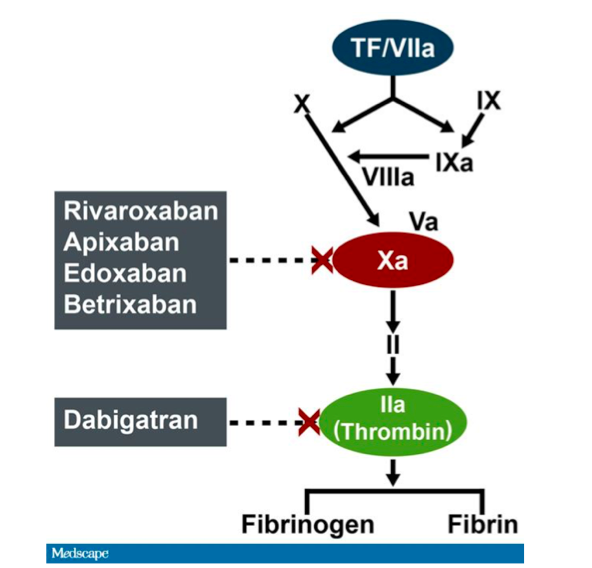
Idarucizumab, a Specific Reversal Agent for Dabigatran: Mode of Action, Pharmacokinetics and Pharmacodynamics, and Safety and Ef

Safety, pharmacokinetics and pharmacodynamics of idarucizumab, a specific dabigatran reversal agent in healthy Japanese volunteers: a randomized study - Yasaka - 2017 - Research and Practice in Thrombosis and Haemostasis - Wiley Online Library
Boehringer Ingelheim submits applications for approval of Idarucizumab, an anti-anticoagulant - Labiotech.eu

Dabigatran Reversal With Idarucizumab in Patients With Renal Impairment | Journal of the American College of Cardiology

Safety, pharmacokinetics and pharmacodynamics of idarucizumab, a specific dabigatran reversal agent in healthy Japanese volunteers: a randomized study - Research and Practice in Thrombosis and Haemostasis

Idarucizumab and factor Xa reversal agents: role in hospital guidelines and protocols - ScienceDirect

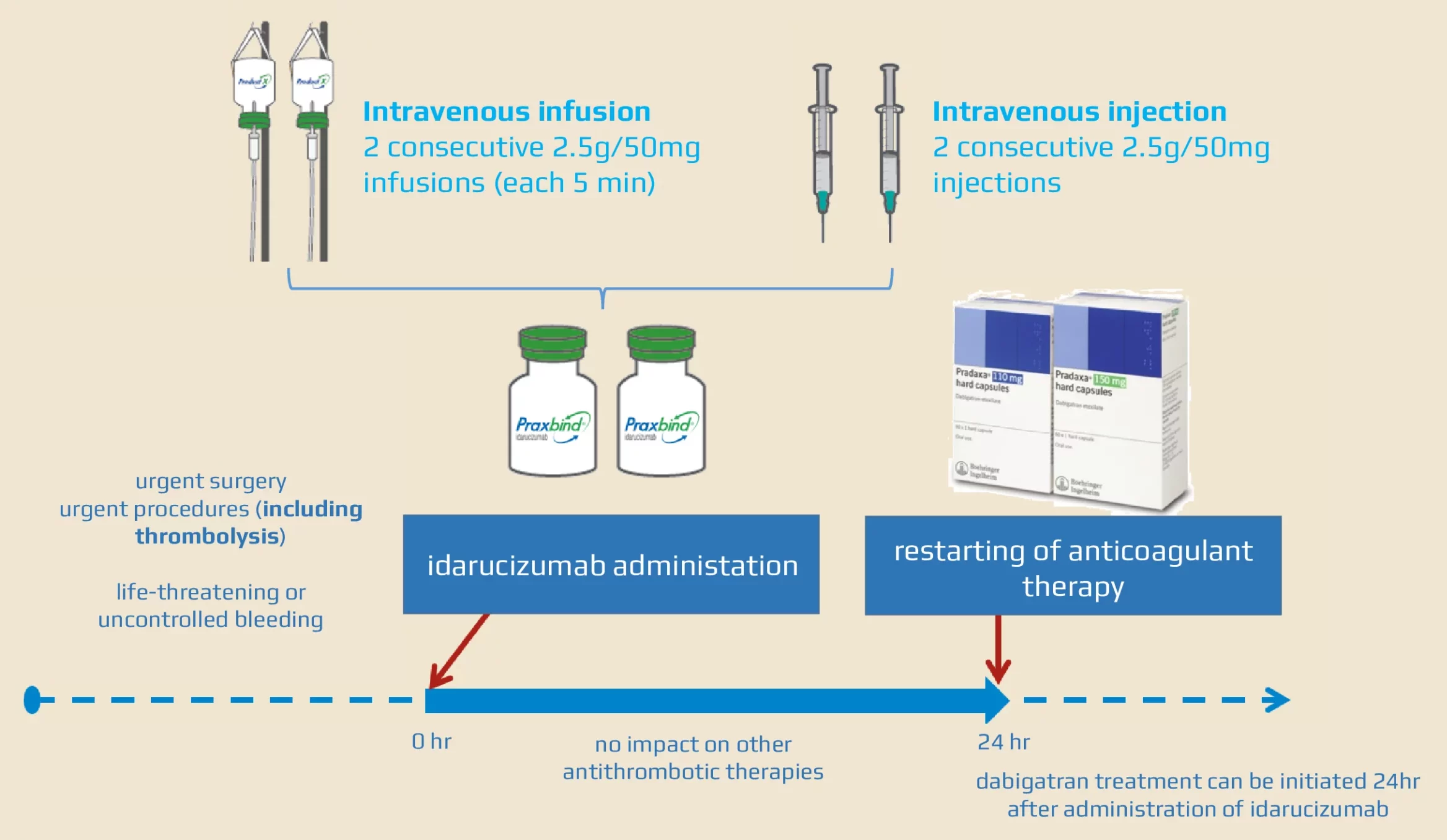
These highlights do not include all the information needed to use PRAXBIND safely and effectively. See full prescribing information for PRAXBIND. PRAXBIND® (idarucizumab) injection, for intravenous useInitial U.S. Approval: 2015