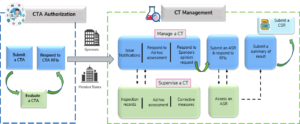
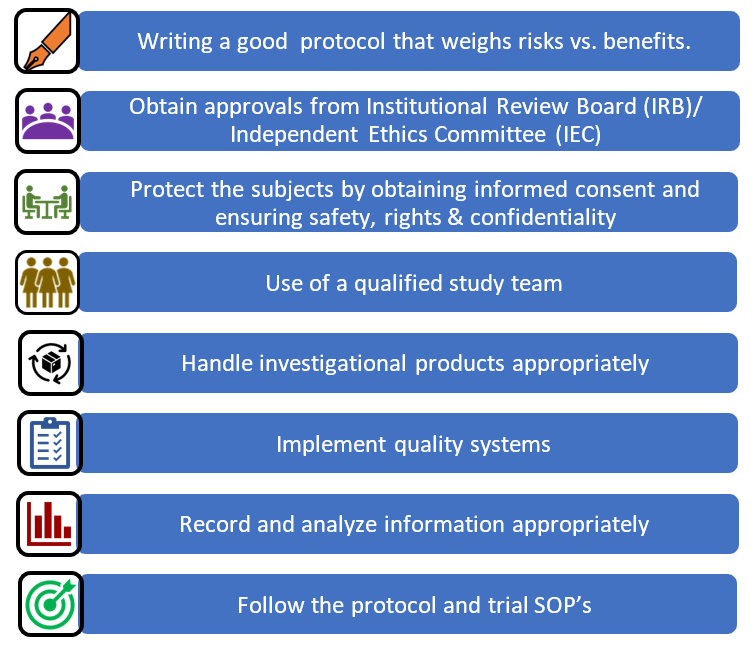
Training log for new clinical research associates (CRAs). P&P, policies... | Download Scientific Diagram

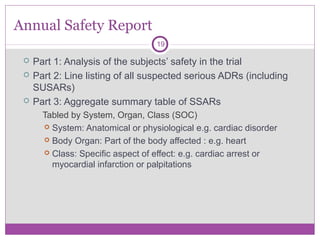
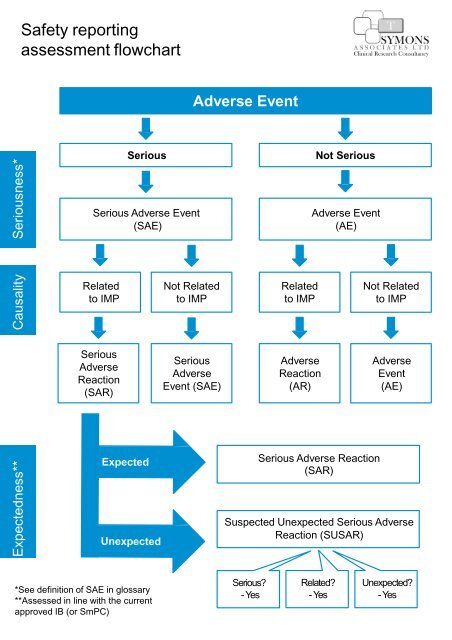
SOLVED: In addition to serious adverse event reporting, what other reporting requirements exist for HGT trials? Quarterly enrollment statistics from all clinical trial sites Annual reports within 60 days of the anniversary